NS0&SP2/0 HCD resDNA Detection Kit
$1,899.00
1fg/μL ultra-sensitive NS0&SP2/0 residual DNA quantitation kit engineered for murine myeloma cell-derived therapeutic protein production—supports both high molecular weight and sheared DNA detection. Features 1.5-hour total rapid TaqMan qPCR test, 3-step 20-minute sample prep with room-temperature stable components, 1fg/μL-300pg/μL broad linear range, and <15% CV for key concentrations. 70-130% recovery rate for all sample types, fully GMP-compliant for biopharm QC testing. Request your custom quote today!
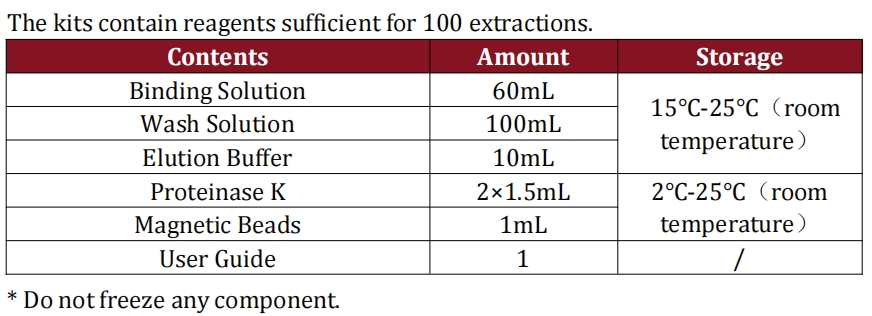
| Catalog number: | D24011203 |
|---|---|
| Qty: | 100 Reactions |
| Include | Assay Kit only |
Description
resDNASEQ NS0&SP2/0 Residual DNA Quantitation Kit – TaqMan qPCR for Murine Myeloma Therapeutic Protein Production
NS0&SP2/0 murine myeloma cells are the gold standard cell lines for commercial therapeutic protein production and biomedical research, valued for their high-yield recombinant protein expression capabilities. However, the manufacturing and purification processes for these biotherapeutics carry the risk of host cell DNA contamination from NS0&SP2/0 cells, making accurate, sensitive quantitation of this residual DNA a critical step for regulatory compliance and product safety. The NS0&SP2/0 Residual DNA Quantitation Kit from Zhengzhou Ducky Bio is a murine myeloma cell-exclusive TaqMan real-time qPCR assay, purpose-built to detect and quantify trace NS0&SP2/0 host cell DNA. Unlike generic residual DNA kits, it is optimized for the unique genomic traits of NS0&SP2/0 cells and supports quantitation for both high molecular weight and sheared DNA, delivering reliable results for all sample types from in-process material to final therapeutic protein products.
1.5-Hour Rapid Workflow with Simplified Room-Temp Sample Prep
We’ve optimized this kit for the high-throughput demands of therapeutic protein manufacturing QC labs, with a streamlined workflow that cuts total test time to just 1.5 hours—a transformative improvement for biopharm production timelines. Sample preparation requires only 3 simple steps and just 20 minutes to complete, with no complex protocols or specialized technical training needed for lab personnel.
A key advantage is that all components of the Residual DNA Sample Preparation Kit are stable at room temperature, eliminating the need for costly cold chain storage and shipping, and simplifying inventory management for global biomanufacturing facilities.
The kit also features a single ready-to-use qPCR MIX, eliminating manual pipetting of multiple reagents, reducing the risk of human error, and further accelerating the testing process—critical for scaling therapeutic protein production.
1fg/μL Ultra-Sensitivity with Exceptional Precision & Linearity
Powered by gold-standard TaqMan™ real-time qPCR technology, this kit delivers industry-leading sensitivity for NS0&SP2/0 residual DNA detection, with a Limit of Quantification (LOQ) of 1fg/μL—enabling detection of even trace levels of murine myeloma host cell DNA in therapeutic protein samples. Rigorous validation across six concentration gradients (3fg/μL, 30fg/μL, 300fg/μL, 3pg/μL, 30pg/μL, 300pg/μL) confirms exceptional precision and linearity: CV of each concentration was <15%, the regression coefficient associated with standard solutions was 0.99995, and amplification efficiency reached 100.430%.
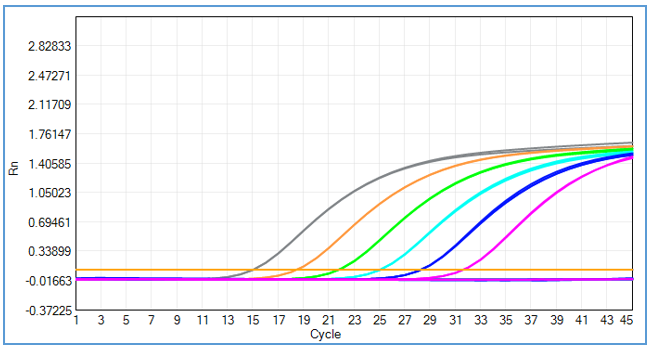

Further validation across five concentrations (0.3fg/μL, 1fg/μL, 3fg/μL, 10fg/μL, 30fg/μL) with 10 multiple wells per concentration showed that the CV of concentration values for samples with 1fg/μL and above was less than 15%, meeting the strict precision requirements of GMP-compliant therapeutic protein QC testing.


Reliable Performance Across All Bioproduction Sample Types
A cornerstone of this NS0&SP2/0 kit is its reliable performance across the full spectrum of bioproduction sample types, including in-process cell culture supernatants, purified therapeutic protein formulations, and samples containing both high molecular weight and sheared NS0&SP2/0 DNA. Spiked sample testing with five concentrations (0.03pg/μL, 0.3pg/μL, 3pg/μL, 30pg/μL, 300pg/μL)—purified with the sample preparation kit and detected with the amplification kit—confirmed that the recoveries of all simulated samples were between 80% and 120%.


For all concentrations in the linear range, the kit achieves a recovery rate of 70-130%, with consistent results regardless of sample processing stage or DNA integrity. This robust performance ensures biomanufacturers can rely on the kit for every step of NS0&SP2/0-derived therapeutic protein production, from process development and scale-up to final product release and regulatory compliance.
NS0&SP2/0-Exclusive Design: Tailored for Murine Myeloma Production
What sets our resDNASEQ NS0&SP2/0 kit apart from generic residual DNA detection kits is its exclusive optimization for NS0 and SP2/0 murine myeloma cells—the primary cell lines for commercial therapeutic protein production. Unlike one-size-fits-all assays that lack specificity for murine myeloma DNA, this kit is engineered to target NS0&SP2/0-specific genomic sequences, avoiding cross-reactivity with other host-cell or environmental DNA and ensuring every quantitation result reflects only true residual NS0&SP2/0 DNA levels. The kit’s streamlined design, rapid workflow, and room-temperature stable components make it seamlessly integrable into GMP-compliant laboratory workflows, while all performance data is fully validated to support regulatory submissions to global authorities including the FDA, EMA, and NMPA. For therapeutic protein manufacturers, this kit is not just a QC tool, but a strategic solution for ensuring product safety and meeting global biopharmaceutical regulatory standards.
Core Application Scenarios: NS0&SP2/0-Derived Bioproduction
This NS0&SP2/0 Residual DNA Quantitation Kit is fully validated for all key stages of NS0&SP2/0 murine myeloma cell-derived bioproduction, with applications tailored exclusively to the needs of therapeutic protein manufacturers and biomedical research labs. It is the ideal solution for in-process QC testing (monitoring residual DNA levels during NS0&SP2/0 cell culture and protein purification), final product quantitation of NS0&SP2/0 host cell DNA in recombinant therapeutic proteins, and process development and scale-up (tracking residual DNA changes as production scales from lab to commercial manufacturing). The kit is also suitable for GMP-compliant regulatory compliance testing, supporting submissions for biotherapeutic products produced using NS0 or SP2/0 cells. Whether manufacturing monoclonal antibodies, fusion proteins, or other recombinant therapeutic proteins, this kit delivers the sensitivity, precision, and efficiency needed to ensure product safety and regulatory compliance.
You May Also Interested
Frequently Asked Questions (FAQ)
1.What is the LOQ of this NS0&SP2/0 residual DNA kit?
Answer: The kit has a Limit of Quantification (LOQ) of 1fg/μL, with CV <15% for all samples at or above this concentration.
2.How long does a full NS0&SP2/0 DNA test take?
Answer: The entire workflow takes just 1.5 hours total—20 minutes for sample preparation and the remaining time for qPCR amplification and quantitation.
3.Do the sample preparation components require cold storage?
Answer: No, all components of the Sample Preparation Kit are stable at room temperature for storage and shipping, no cold chain required.
4.What sample types is this kit compatible with?
Answer: It supports all bioproduction sample types, including in-process cell culture supernatants, purified therapeutic proteins, and samples with high molecular weight or sheared DNA.
5.What is the linear range of this NS0&SP2/0 assay?
Answer: The kit is validated for a linear range from 1fg/μL to 300pg/μL, with consistent CV <15% and 70-130% recovery across all concentrations.
Ready to Streamline Your NS0&SP2/0 Residual DNA Testing?
If you’re manufacturing therapeutic proteins using NS0 or SP2/0 murine myeloma cells, our resDNASEQ NS0&SP2/0 Residual DNA Quantitation Kit delivers the 1fg/μL ultra-sensitivity, 1.5-hour rapid workflow, and murine myeloma-specific design your QC team needs to ensure product safety and regulatory compliance. Contact our dedicated sales and technical support team today to request a custom quote, learn more about bulk pricing options, or get personalized guidance on integrating this assay into your GMP-compliant therapeutic protein production workflow. Let us help you streamline your testing timelines, reduce operational costs, and accelerate your biotherapeutic development—reach out now to get started.
Related products
-
Sample Preparation Kit Ⅱ
$899.00







