Human Residual DNA Fragment Analysis Kit
$3,699.00
30 fg/μL LoD Human Residual DNA Detection Kit with TaqMan qPCR technology. Simultaneously detects 4 DNA fragment sizes (66/150/200/528 bp), 30-minute sample prep, 3-hour total test time. Equipped with UDG enzyme for anti-amplification contamination, 70%-130% recovery rate, and <20% CV. Ideal for biopharmaceutical human host-cell/plasmid DNA fragment QC testing. Get your special quote today!
| Catalog number: | Cat. No. D24011210 |
|---|---|
| Qty: | 100 Reactions |
Description
Better Human DNA Fragment Analysis Kit
Core Unique Advantages
- Fragment-Specific Multiplex Detection: The only kit in its class to simultaneously quantify four key human DNA fragment sizes (66 bp, 150 bp, 200 bp, 528 bp) in a single qPCR workflow—critical for analyzing DNA fragmentation during bioprocessing (e.g., cell lysis, purification). Even the 500+ bp long fragment achieves nearly 100% amplification efficiency, ensuring no fragment is overlooked in your QC testing.
- Ultra-Sensitive Quantitation with Anti-Contamination Design: Boasts a Limit of Detection (LoD) of 30 fg/μL and uniform Limit of Quantification (LOQ) across all four fragments, enabling femtogram-level human residual DNA detection. Integrated UDG enzyme eliminates amplification product contamination—a common pain point in qPCR-based DNA testing—while an internal positive control (IPC) validates the performance of every individual PCR reaction, eliminating false negatives.
- Rapid Streamlined Workflow: No complex sample preparation protocols or specialized training required—30 minutes only for sample prep, with a single ready-to-use qPCR reagent mix to reduce hands-on time and human error. The entire assay (sample prep + qPCR amplification + fragment quantitation) is completed in just 3 hours, significantly accelerating bioprocess QC timelines compared to traditional fragment analysis methods (e.g., gel electrophoresis).
- Exceptional Accuracy & Reproducibility: Delivers a recovery rate of 70%-130% across all linear range concentrations, with a coefficient of variation (CV) of <20% for all four fragment sizes—ensuring reliable results even in complex biotherapeutic matrices. Amplification efficiencies for all fragments range from 99% to 101%, guaranteeing consistent, batch-to-batch performance for routine QC and process development.
Kit Performance & Validation
1.Amplification Efficiency: 99%-101% across all four fragment sizes (66 bp to 528 bp), including long 500+ bp fragments that challenge most qPCR assays.
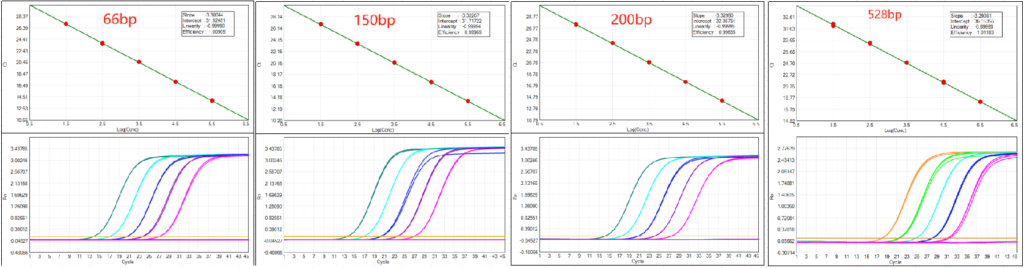
2.Accuracy: Average detection concentration aligns closely with theoretical values across 30 fg/μL to high-concentration samples, with accuracy deviation within an acceptable range for regulatory QC.
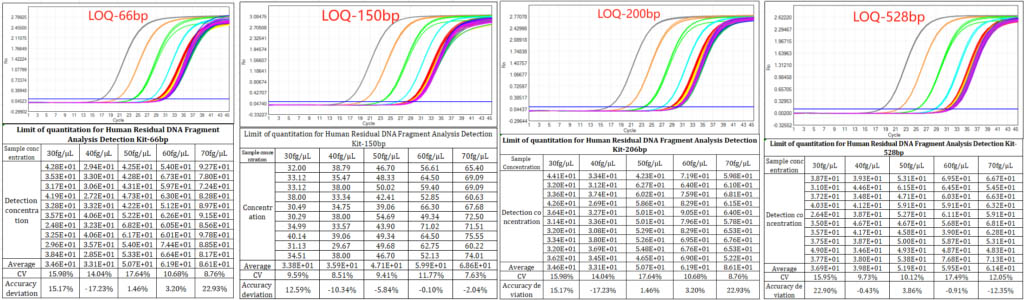
3.Precision: CV values as low as 8.56% for key fragment sizes (70 fg/μL 66 bp), with all tested concentrations showing CV <20%—meeting strict GMP requirements for data reproducibility.
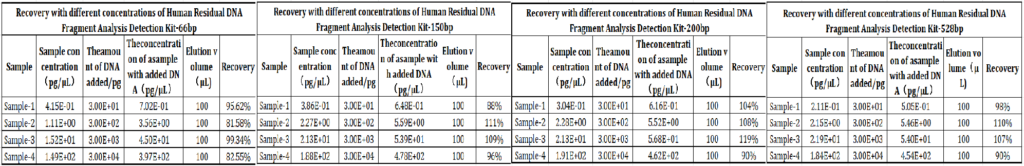
4.Recovery: 70%-130% recovery rate for spiked samples in diverse bioprocess matrices (cell culture supernatants, purified protein formulations), validating performance in real-world testing scenarios.
Why Choose Our Human DNA Fragment Analysis Kit?
Key Applications
- Human cell line-derived biotherapeutic production QC (monoclonal antibodies, fusion proteins)
- Human plasmid DNA fragment analysis in bioprocessing
- In-process sample testing for human host-cell DNA residuals (cell culture, purification, formulation)
- Final biotherapeutic product human residual DNA fragment quantitation
- Vaccine manufacturing (human cell line-based) residual DNA fragment analysis
- Bioprocess development and scale-up (tracking DNA fragmentation during process optimization)
- Regulatory compliance testing for human residual DNA in biopharmaceuticals
Technical Specifications
| Parameter | Specification |
|---|---|
| Catalog Number | D24011210 |
| Reaction Capacity | 100 Reactions |
| Detection Technology | TaqMan Real-Time qPCR with UDG Anti-Contamination |
| Detected Fragment Sizes | 66 bp, 150 bp, 200 bp, 528 bp (simultaneous detection) |
| Limit of Detection (LoD) | 30 fg/μL (for all fragments) |
| Limit of Quant (LOQ) | 30 fg/μL (for all fragments) |
| Amplification Efficiency | 99% – 101% (all four fragments, including 528 bp) |
| Recovery Rate | 70% – 130% (linear range concentrations) |
| Coefficient of Variation (CV) | <20% (all tested concentrations) |
| Sample Preparation Time | 30 Minutes |
| Total Assay Time | 3 Hours (sample prep + qPCR + quantitation) |
| Key Components | UDG enzyme, Internal Positive Control (IPC), Human DNA Control, Single qPCR MIX |
| Sample Compatibility | Cell culture supernatants, bulk drug substances, final biotherapeutic formulations |
| Operation Feature | Single qPCR reagent mix, 5 dilutions for standard curve generation |
| Quality Control | Batch-to-batch performance validation, detailed test data included |
You May Also Interested
FAQ
1.What fragment sizes does this human residual DNA kit detect?
2.What is the detection limit of this human residual DNA fragment analysis kit?
3.How does this kit prevent qPCR amplification contamination?
4.How long does a full human residual DNA fragment analysis take with this kit?
5.Does the kit include a control for validating PCR reaction performance?
6.What sample types is this kit suitable for?
Related products
-
NS0&SP2/0 HCD resDNA Detection Kit
$1,899.00 -
HEK293 ResDNA Quantitation Kit
$1,899.00