E.coli Residual DNA Quantitation kit
$1,899.00
Buy Our High-sensitivity E. coli residual DNA detection kit (LoD 1 fg/μL). Rapid TaqMan™ qPCR assay with 3-step sample prep (20 mins), 1.5-hour for total test. Complies with WHO standards (≤10 ng per therapeutic dose), featuring 70%-130% recovery rate and CV <30%. Easy to use with room temperature storable sample preparation components. Get Free Trial & discount quote now!
| Catalog number: | D24011201 |
|---|---|
| Qty: | 100 Reactions |
| Include | Assay Kit only |
Description
High Precision E.coli Residual DNA Detection Kit From China
Rapid Results & Streamlined Workflow
1. Say goodbye to complex sample preparation and lengthy wait times. Our kit requires only 3 simple steps for sample prep (completing in just 20 minutes).
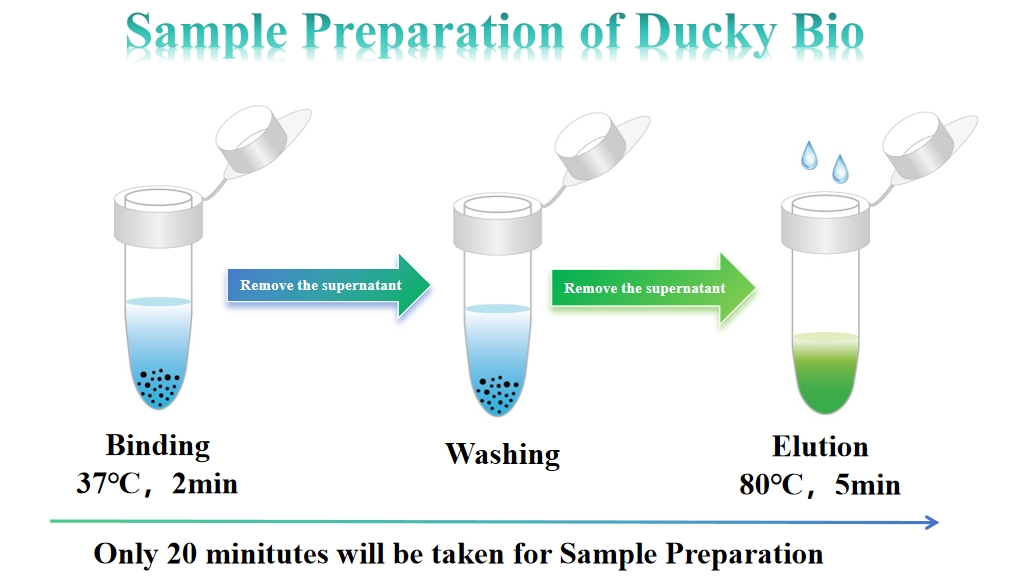
2. All components of the Sample Preparation Kit can be stored at room temperature—eliminating the need for costly cold storage and simplifying inventory management.
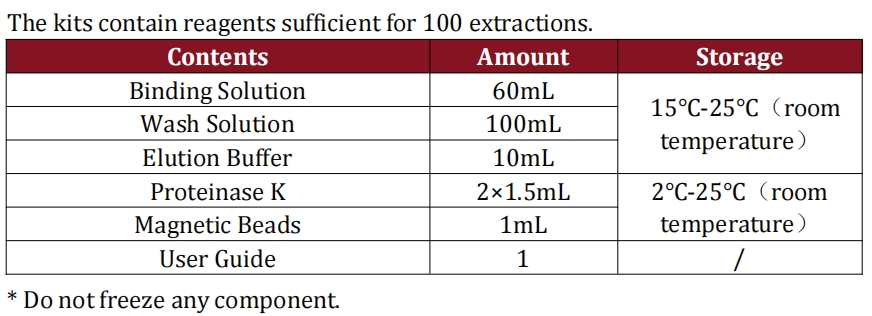
3.Only one reagent for qPCR MIX, and the entire test (from sample prep to results) takes just 1.5 hours—significantly boosting your QC workflow efficiency.

Exceptional Sensitivity & Accuracy
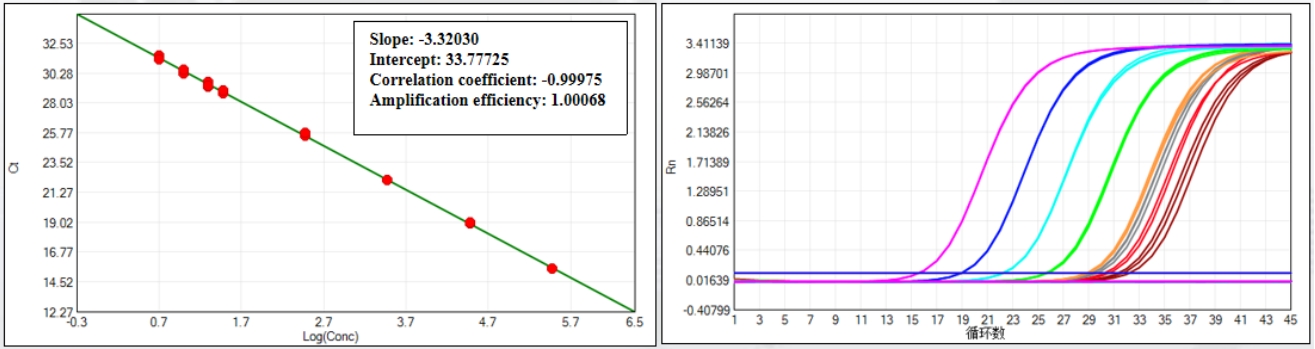
Leveraging proven TaqMan™ qPCR technology, this kit achieves a Limit of Detection (LoD) of 1 fg/μL and a Limit of Quantification (LoQ) of 5 fg/μL—enabling femtogram-level E. coli DNA detection that outperforms industry standards. With a regression coefficient of ≥0.999 for standard solutions and coefficient of variation (CV) <30% across all tested concentrations (5 fg/μL to 300 pg/μL), it ensures consistent, trustworthy data for critical QC decisions.
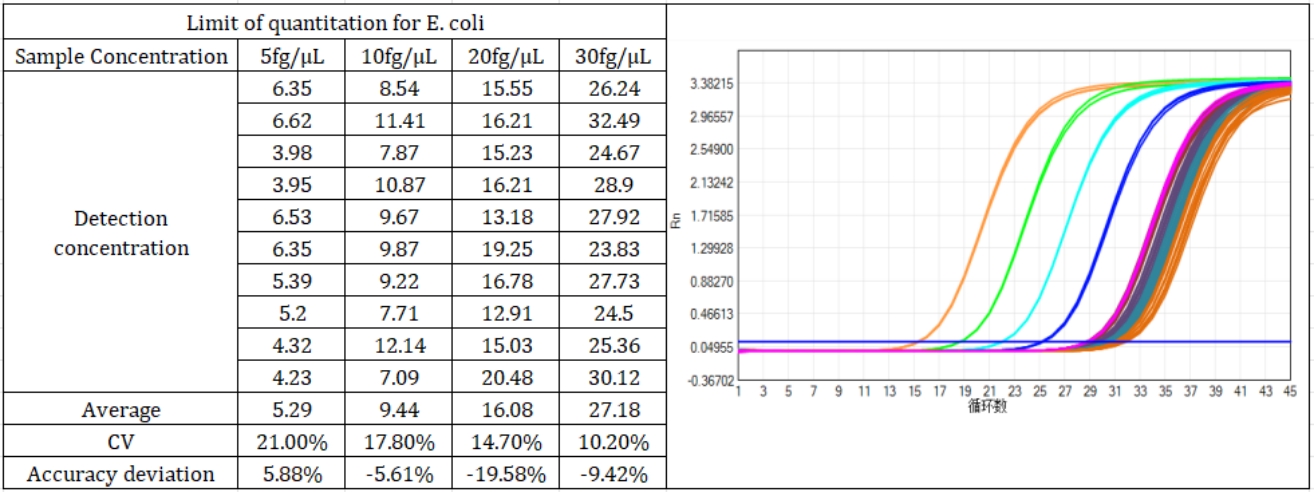
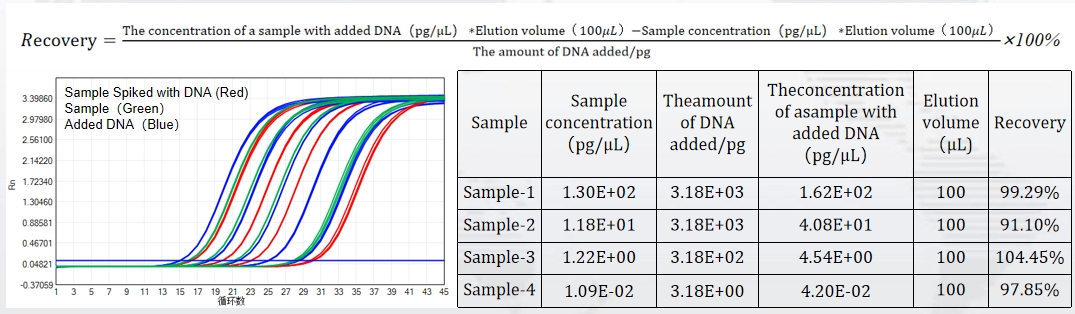
The kit offers a broad linear range from 5 fg/μL to 300 pg/μL, covering the full spectrum of residual DNA concentrations encountered in bioproduction. Spiked sample tests confirm a recovery rate between 70% and 130%—ensuring accurate quantification even in complex matrices. With an amplification efficiency of 100.068%, it delivers perfect amplification curves and consistent results batch after batch.
Why Choose Our E. coli Residual DNA Kit?
Applications
- Biopharmaceutical production (monoclonal antibodies, recombinant proteins, fusion proteins)
- Viral and subunit vaccine manufacturing
- E. coli-derived production cell line quality control
- Plasmid DNA residual quantitation in bioprocesses
- Regulatory compliance testing (aligns with WHO, FDA, and EMA guidelines)
- Bioprocess development and scale-up QC
You May Also Interested
- CHO residual DNA quantitation kit
- NS0&SP20 residual DNA detection kit
- E1A&SV40LTA qPCR residual DNA kit
- Human residual DNA test kit
- HEK293 residual DNA quantification kit
- Pichia Pastoris residual DNA quantification kit
- Vero residual DNA quantification kit
Frequently Asked Questions (FAQs)
1.What is the detection limit of the resDNASEQ E. coli Residual DNA Quantitation Kit?
Answer: The kit offers an ultra-high sensitivity with a Limit of Detection (LoD) of 1 fg/μL and a Limit of Quantification (LoQ) of 5 fg/μL—making it capable of femtogram-level E. coli DNA detection, which exceeds global regulatory requirements.
2.How long does it take to complete a full E. coli residual DNA test with this kit?
Answer: The entire process takes only 1.5 hours. Sample preparation requires just 20 minutes (3 straightforward steps), and the qPCR amplification and quantification step follows seamlessly—saving valuable time compared to traditional kits.
3.Is this kit suitable for vaccine production residual DNA detection?
Answer: Yes, it is widely used in vaccine manufacturing QC processes, including viral vaccines and subunit vaccines. It effectively quantifies E. coli residual DNA in vaccine substrates and final products, supporting compliance with WHO and regional regulatory standards.
4.Do the sample preparation components require cold storage?
Answer: No—all components of the Sample Preparation Kit can be stored at room temperature, which simplifies storage and transportation, reduces cold chain costs, and ensures stability under standard lab conditions.
5.Does this kit comply with WHO guidelines for residual E. coli DNA?
Answer: Absolutely. The kit is specifically developed to meet the sensitivity requirement defined by WHO (10 ng E. coli DNA per therapeutic dose), making it a reliable choice for regulatory compliance in biopharmaceutical and vaccine production.
Related products
-
HEK293 ResDNA Quantitation Kit
$1,899.00








