E1A&SV40LTA Residual Host Cell DNA Assay Kit
$2,999.00
4 copies/μL ultra-sensitive E1A&SV40LTA residual DNA quantitation kit engineered for HEK293T cell/gene therapy and lentiviral vector production. Features 1.5-hour total rapid TaqMan qPCR test, dual gene simultaneous quantitation (E1A/SV40LTA), 4 copies/μL LOQ, room-temperature sample prep components, and <20% CV for key concentrations. 70-130% recovery rate, fully validated for HEK293T-derived biotherapeutics and GMP-compliant QC testing. Request your custom quote today!
| Catalog number: | Cat. No. D24011210 |
|---|---|
| Qty: | 100 Reactions |
Description
E1A & SV40 Large T Antigen (LTA) Residual DNA Quantitation Kit
HEK293T is a very common expression vector in cell therapy, gene therapy and biopharmaceuticals, and is widely used in the production of proteins, vaccines, anticancer agents and recombinant adenovirus coatings. HEK 293T cells carry specific transformation sequences that are known to have potential safety risks, such as Adv E1A sequences and SV40 large T antigen sequences. Therefore, relevant regulations require that when HEK293T cells are used to prepare lentiviral vectors in products, a method with sufficient sensitivity and specificity must be used to detect the residues of adenovirus E1 and SV40LTA antigen sequences.
Ultra-Fast 1.5-Hour Workflow with Simplified Sample Prep
We’ve optimized this kit for the high-throughput demands of cell and gene therapy QC labs, with a streamlined workflow that cuts total test time to just 1.5 hours—the fastest E1A&SV40LTA detection assay on the market for HEK293T samples. Sample preparation requires only 3 simple steps and 20 minutes.

With no complex protocols or specialized technical training needed. All components of the sample preparation kit are stable at room temperature for storage and shipping.
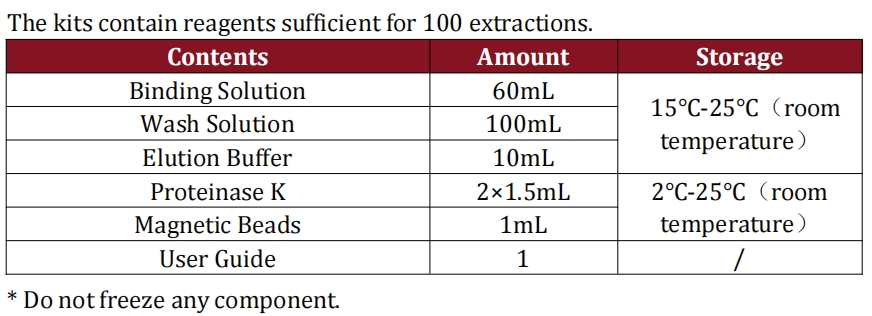
Eliminating the need for costly cold chain logistics and simplifying inventory management for global biomanufacturing facilities. The kit also features a single ready-to-use qPCR reagent mix, eliminating manual pipetting of multiple components, reducing human error, and further accelerating testing timelines—critical for scaling cell and gene therapy production.
Unmatched Ultra-Sensitivity for Dual Gene Quantitation
Powered by gold-standard TaqMan™ real-time qPCR technology, this kit delivers industry-leading ultra-sensitivity for simultaneous E1A and SV40LTA quantitation—a critical advantage for HEK293T residual DNA testing. It achieves a Limit of Detection (LOD) of 0.4 copies/μL and a Limit of Quantification (LOQ) of 4 copies/μL for both E1A and SV40LTA, enabling detection of even trace levels of these transforming sequences in HEK293T-derived samples (e.g., lentiviral vectors). Rigorous validation across seven concentration gradients (4 to 4×10⁶ copies/μL) confirms exceptional precision and linearity: E1A shows a regression coefficient (R²) of 0.99993, amplification efficiency of 99.753%, and CV < 10% for all concentrations.

SV40LTA delivers an R² of 0.99995, amplification efficiency of 100.737%, and CV < 15% across all concentrations.

For concentrations ≥4 copies/μL, both target genes show CV < 20%, meeting the strict precision requirements of GMP-compliant QC testing..




Reliable Performance in Real-World Bioproduction Samples
Reliable performance in complex HEK293T bioproduction matrices is the cornerstone of this E1A&SV40LTA kit, with validated results for both simulated and actual bioprocess samples (including cell culture supernatants, lentiviral vectors, and recombinant protein formulations). Spiked sample testing with 4 real HEK293T-derived samples confirms a 90-100% recovery rate for E1A DNA,And the same 90-100% recovery for SV40LTA DNA. 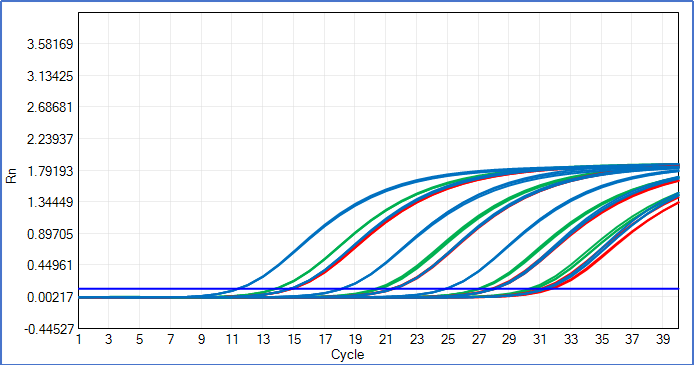



After purification with the DuckyBio Host Cell Preparation Kit. The kit achieves a 70-130% recovery rate across all linear range concentrations for both target genes, with strong resistance to matrix interference—ensuring accurate quantitation regardless of sample type, whether testing in-process HEK293T cell culture or final cell/gene therapy products. This robust performance makes it a trusted tool for every stage of HEK293T bioproduction, from process development to final product release.
Dual-Gene Exclusive Design: Superior to Generic HEK293T Kits
What sets this kit apart from generic HEK293T residual DNA detection kits is its exclusive dual-gene optimization for E1A and SV40LTA—the two high-risk transforming sequences unique to HEK293T cells. Rather than a one-size-fits-all solution, it features custom-designed primer-probes for both E1A and SV40LTA, enabling simultaneous quantitation of both target sequences in a single qPCR reaction, with no cross-reactivity with other host-cell or environmental DNA. The kit is specifically designed for use with the DuckyBio Host Cell Preparation Kit, creating a seamless end-to-end workflow for HEK293T residual DNA testing—from sample purification to dual-gene quantitation—ensuring maximum accuracy and consistency. Every kit undergoes rigorous batch-to-batch validation, with detailed performance data included to support regulatory submissions, and our dedicated technical support team is available to assist with HEK293T-specific QC workflow optimization. For cell and gene therapy manufacturers, this kit is not just a QC tool, but a strategic safety solution for mitigating HEK293T-related risks.
Core Application Scenarios: HEK293T-Derived Cell & Gene Therapy
This E1A&SV40LTA Residual DNA Quantitation Kit is fully validated for all key stages of HEK293T-based bioproduction, with application scenarios tailored exclusively to the needs of cell therapy, gene therapy, and biopharmaceutical manufacturers. It is the ideal solution for in-process QC testing (including HEK293T cell culture, lentiviral/adenoviral vector production, and anticancer agent manufacturing), final product quantitation of E1A&SV40LTA residual DNA in cell/gene therapy products, and process development/scale-up (tracking target sequence levels as HEK293T production scales). The kit is also suitable for GMP-compliant regulatory compliance testing, supporting submissions to global authorities including the FDA, EMA, and NMPA for HEK293T-derived biotherapeutics. Whether you’re producing lentiviral vectors for ex vivo gene therapy, recombinant proteins, or cancer immunotherapies, this kit delivers the ultra-sensitivity and dual-gene specificity you need to ensure product safety and regulatory compliance.
You May Also Interested
Frequently Asked Questions (FAQ)
1.What is the sensitivity of this E1A&SV40LTA kit?
Answer: It achieves an ultra-high LOD of 0.4 copies/μL and LOQ of 4 copies/μL for both E1A and SV40LTA, enabling detection of trace residual DNA in HEK293T-derived samples.
2.How long does a full E1A&SV40LTA test take?
Answer: The entire workflow takes just 1.5 hours total—20 minutes for sample preparation and the remaining time for qPCR amplification and dual-gene quantitation.
3.Does the kit require cold chain storage for sample prep components?
Answer: No, all sample preparation kit components are stable at room temperature for storage and shipping, eliminating costly cold chain logistics.
4.Is this kit compatible with other sample preparation kits?
Answer: It is optimized for use with the DuckyBio Host Cell Preparation Kit for maximum accuracy, and can be adapted for use with other standard prep kits with minor adjustments.
5.Can this kit be used for large-scale bioproduction QC?
Answer: Yes, its 1.5-hour rapid workflow and high precision (CV <20% for key concentrations) meet the high-throughput needs of large-scale GMP-compliant cell/gene therapy production lines.
Ready to Optimize Your HEK293T Residual DNA Testing?
If you’re manufacturing HEK293T-derived cell therapy, gene therapy, or lentiviral vector products, our resDNASEQ E1A&SV40LTA Residual DNA Quantitation Kit delivers the ultra-sensitivity, dual-gene specificity, and rapid workflow your QC team needs to mitigate safety risks and meet global regulatory standards. Contact our dedicated sales and technical support team today to request a custom quote, learn more about bulk pricing options, or get personalized guidance on integrating this assay into your HEK293T GMP workflow. Let us help you streamline your testing timelines, ensure product safety, and accelerate your cell/gene therapy development—reach out now to get started.
Related products
-
NS0&SP2/0 HCD resDNA Detection Kit
$1,899.00 -
Sample Preparation Kit Ⅱ
$899.00 -
Pichia Pastoris resDNA Assay Kit
$1,899.00 -
Vero Residual HCD Quantitation Kit
$1,899.00








