HEK293 ResDNA Quantitation Kit
$1,899.00
Note: Price not include shipment & duty, contact us to get full quote.
30fg/μL ultra-sensitive HEK293 residual DNA testing kit engineered for gene therapy and recombinant protein production—critical for detecting tumorigenic/retroviral DNA sequences in HEK293-derived biotherapeutics. Features 30-minute streamlined sample prep, 2-hour total TaqMan qPCR test, 30fg/μL-300pg/μL broad linear range (R²≈0.99989), 100.192% amplification efficiency, and CV<20% for key concentrations. 70-130% recovery rate for simulated/actual samples, fully GMP-compliant for biopharm and gene therapy QC testing. Request your custom quote today!
| Catalog number: | D24011205 |
|---|---|
| Qty: | 100Reactions |
Description
HEK293 Residual DNA Quantitation Kit
Human Embryonic Kidney (HEK293) cells are the workhorse of modern biopharmaceutical production, playing a pivotal role in manufacturing recombinant therapeutic proteins and viral vectors for gene therapy. However, this widely adopted cell line poses a unique safety challenge: residual HEK293 DNA may contain tumorigenic genetic sequences and retroviral elements that carry transmission risks to human recipients. This makes accurate, sensitive quantitation of HEK293 residual DNA an indispensable step for regulatory compliance and biotherapeutic product safety. The resDNASEQ HEK293 Residual DNA Quantitation Kit from Zhengzhou Ducky Bio is a HEK293 cell line-exclusive TaqMan™ real-time qPCR assay, engineered specifically to address the safety and quality control needs of gene therapy and recombinant protein manufacturers. Unlike generic residual DNA kits that lack HEK293 specificity, this assay delivers ultra-sensitive and precise quantitation of HEK293 host-cell DNA, ensuring your biotherapeutics meet the strictest global safety and regulatory standards.
Ultra-Sensitive & Precise Detection for HEK293 Low Residue Requirements
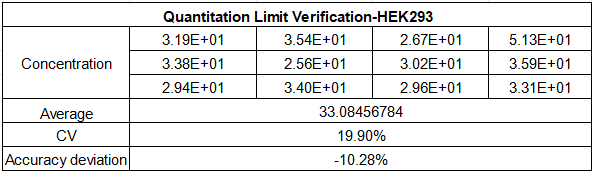
Our HEK293 kit sets a new benchmark for sensitivity and precision in HEK293 residual DNA testing, boasting a Limit of Quantification (LOQ) of 30 fg/μL and a broad linear dynamic range from 30 fg/μL to 300 pg/μL—covering the full spectrum of residual DNA concentrations encountered in HEK293 bioproduction processes. Validated across five key concentration gradients, the assay achieves a regression coefficient (R²) of nearly 0.99989 and an amplification efficiency of 100.192%, generating consistent, reproducible amplification curves for every sample.
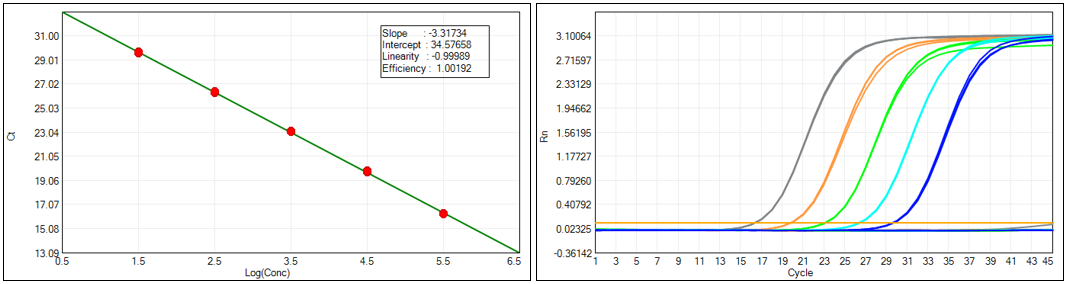
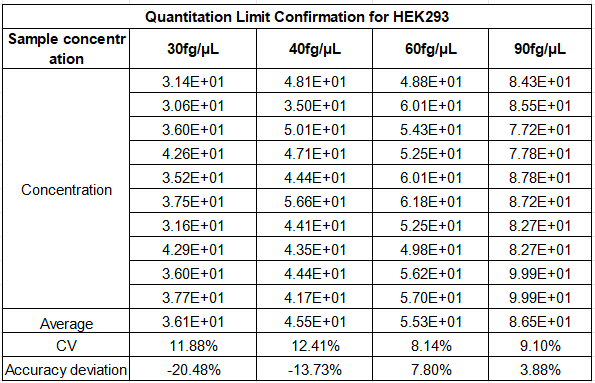
What’s more, it delivers exceptional precision with CV values as low as 8.14% for mid-range concentrations and less than 20% at the 30 fg/μL low limit—far below the 30% industry threshold—with an accuracy deviation within 30% for all tested concentrations. This level of precision ensures reliable detection of even trace amounts of HEK293 DNA, which is critical for mitigating the tumorigenic and retroviral risks associated with HEK293-derived biotherapeutics.
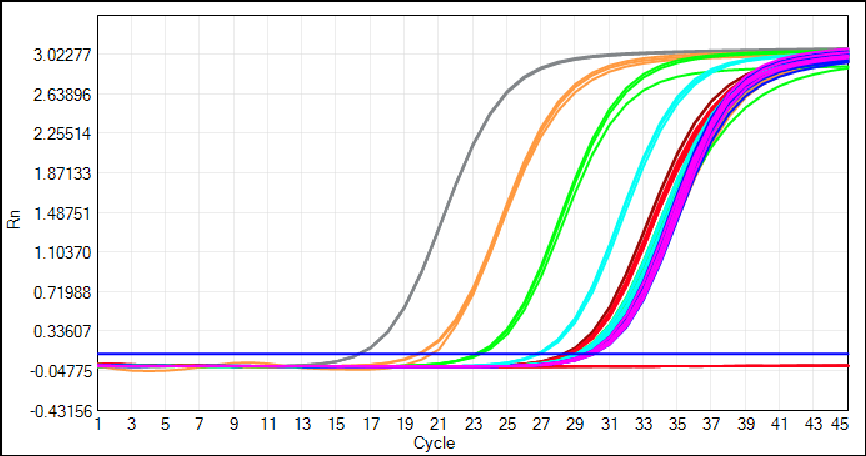
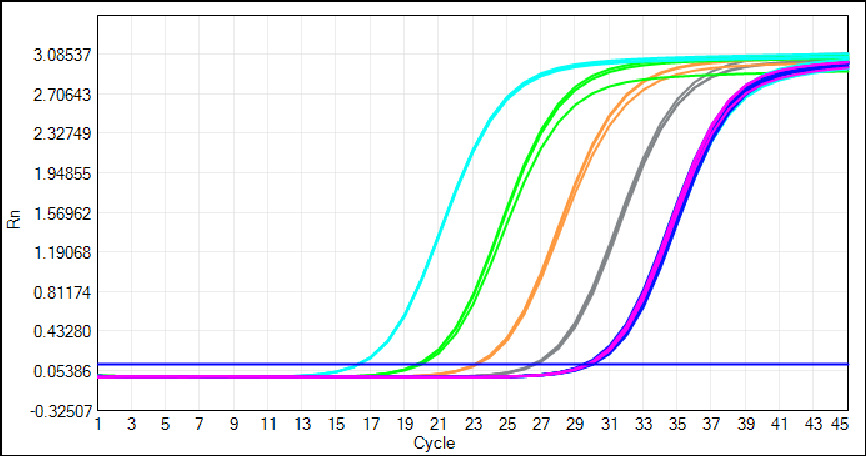
2-Hour Rapid Workflow to Simplify GMP Laboratory Operations
We have optimized the HEK293 kit for seamless integration into GMP-compliant laboratory workflows, prioritizing speed and ease of use without compromising analytical performance. Sample preparation requires only 3 simple steps and 30 minutes—no complex protocols or specialized professional training are needed.

And the kit features a single ready-to-use qPCR MIX that eliminates hands-on pipetting errors and reduces laboratory processing time. The entire assay, from sample preparation to quantitation results, is completed in just 2 hours—a transformative improvement for high-throughput QC teams in gene therapy and recombinant protein production facilities.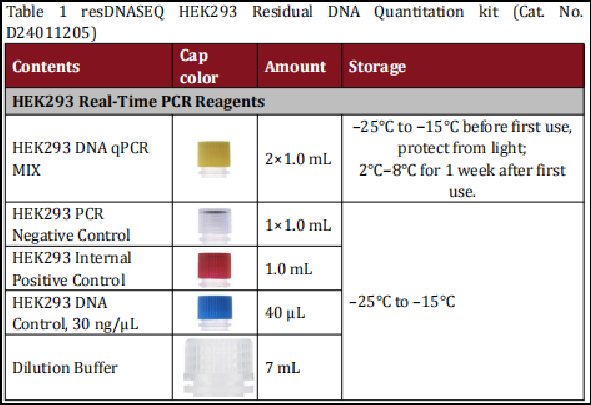

All kit components are designed for convenient storage and handling, with no costly cold chain requirements for sample prep reagents, simplifying inventory management and reducing logistical costs for global biopharmaceutical manufacturers.
High Recovery Rate & Matrix Interference Resistance for Real Production Samples
Reliable performance in real-world bioproduction matrices is the cornerstone of our HEK293 kit, with a validated recovery rate of 70-130% for both simulated HEK293 DNA samples and actual bioprocess samples, including cell culture supernatants, purified protein formulations, and viral vector preparations.
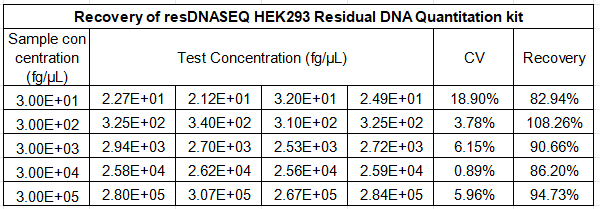
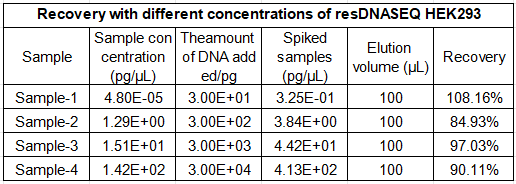
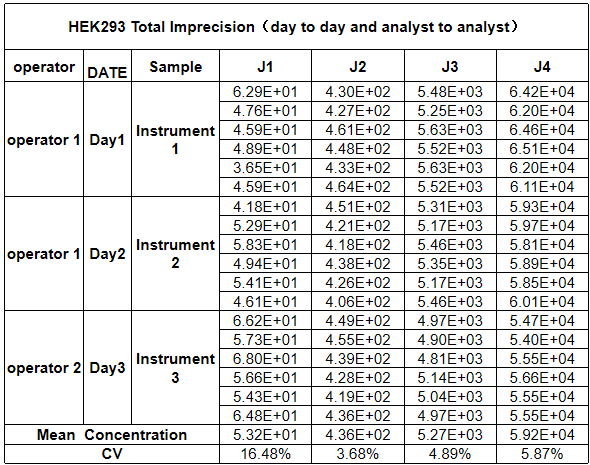
Spiked sample testing confirms the kit’s strong resistance to matrix interference, ensuring accurate quantitation regardless of sample type—whether you are testing in-process cell culture material or final gene therapy products. Additionally, both intra-run and total precision show CV values of less than 30% for all concentrations, meeting the strict GMP requirements for batch-to-batch consistency and regulatory data integrity. This robust performance makes the kit a trusted analytical tool for every stage of HEK293 bioproduction, from process development and scale-up to final product QC testing.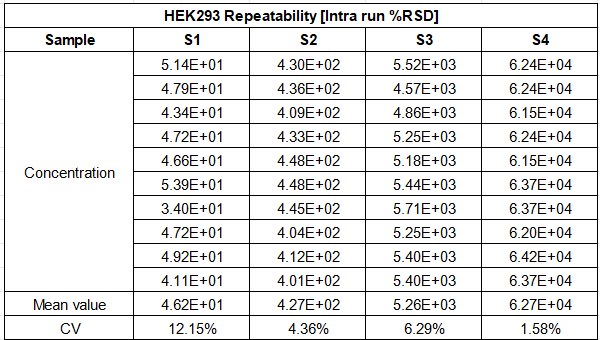


HEK293-Exclusive Design: Superior to Universal Detection Kits
What sets our resDNASEQ HEK293 kit apart from generic residual DNA detection kits is its exclusive optimization for HEK293 cell line DNA—we do not offer a one-size-fits-all solution, but a specialized assay tailored to the unique genomic characteristics of HEK293 cells and the specific safety risks they present. The kit is engineered to target HEK293-specific DNA sequences, avoiding cross-reactivity with other host-cell or environmental DNA, and ensuring that every quantitation result reflects only the true levels of residual HEK293 DNA. As a leader in residual DNA testing solutions for the biopharmaceutical industry, we provide more than just a kit: every order includes detailed batch validation data to support your regulatory submissions, and our dedicated technical support team is available to assist with HEK293-specific QC workflow optimization. For gene therapy and recombinant protein manufacturers, this kit is not just a QC tool, but a strategic safety and compliance solution for HEK293-derived biotherapeutics.
Core Application Scenarios: HEK293 Bioproduction & Gene Therapy QC
Our HEK293 Residual DNA Quantitation Kit is fully validated for all key stages of HEK293 bioproduction, with application scenarios tailored to the unique needs of gene therapy and recombinant protein manufacturing. It is the ideal solution for in-process QC testing (including cell culture, purification, and viral vector production), final product quantitation of HEK293 residual DNA in therapeutic proteins and gene therapy vectors, and process development and scale-up (tracking residual DNA levels during HEK293 production scale-up). The kit is also suitable for GMP-compliant regulatory compliance testing, supporting submissions to global regulatory authorities including the FDA, EMA, and NMPA for HEK293-derived biotherapeutic products. Whether you are producing monoclonal antibodies, fusion proteins, or viral vectors for ex vivo/in vivo gene therapy, this kit delivers the sensitivity and precision you need to ensure product safety and regulatory compliance.
You May Also Interested
Technical Specifications
| Parameter | Specification |
|---|---|
| Catalog Number | D24011205 |
| Reaction Capacity | 100 Reactions |
| Detection Technology | TaqMan Real-Time Quantitative PCR (qPCR) |
| Limit of Quantification (LOQ) | 30 fg/μL |
| Linear Dynamic Range | 30 fg/μL – 300 pg/μL |
| Regression Coefficient (R²) | ≈0.99989 |
| Amplification Efficiency | 100.192% |
| Recovery Rate | 70% – 130% (simulated & actual bioprocess samples) |
| Coefficient of Variation (CV) | <20% for key concentrations; <30% for all tested concentrations |
| Sample Preparation Time | 30 Minutes (3 simple steps) |
| Total Assay Time | 2 Hours (sample prep + qPCR + quantitation) |
| qPCR Reagent Feature | Single ready-to-use qPCR MIX |
| Precision | Within-run & total precision CV <30% |
| Sample Compatibility | HEK293 cell culture supernatants, recombinant protein formulations, gene therapy viral vectors, bulk biotherapeutic substances |
| Compliance | GMP-compliant for biopharm/gene therapy manufacturing QC |
| Key Design Feature | HEK293-specific sequence targeting (no cross-reactivity) |
Frequently Asked Questions (FAQ)
1. Is specialized laboratory equipment required to use this assay?
Answer: No specialized equipment is needed. The assay is compatible with all commercial real-time qPCR instruments, and sample preparation only requires basic lab tools. Its simple 3-step prep process also eliminates the need for advanced technical training.
2. How should the kit components be stored to maintain performance?
Answer: Sample preparation components are stable at room temperature for storage and shipping, so no cold chain is required. qPCR reagents should be stored at -15°C to -25°C and can be kept at 2-8°C for up to 1 week after first use without losing performance.
3. Is this assay suitable for testing viral vectors used in gene therapy?
Answer: Yes. It is fully validated for viral vector preparations, with strong resistance to matrix interference and a 70%-130% recovery rate, making it ideal for gene therapy viral vector QC testing.
4. Can the assay be used for both small-scale and large-scale testing?
Answer: Yes. Its flexible reaction capacity fits small-batch sample testing in research labs. Meanwhile, its high precision and GMP compliance also meet the high-throughput testing needs of large-scale bioproduction lines.
5. Does the kit include controls to ensure test accuracy?
Answer: Yes. Each kit comes with internal positive controls and HEK293 DNA standards, which help validate reaction performance and avoid false negative or positive results, ensuring data integrity for regulatory submissions.
6. What is the typical turnaround time for a full test with this kit?
Answer: The entire testing process takes just 2 hours total—30 minutes for sample preparation and the remaining time for qPCR amplification and quantitation—significantly faster than traditional assays.
7. Can this assay be used for both small-scale laboratory testing and large-scale bioproduction QC?
Answer: Yes, it is designed to adapt to both small-scale laboratory research and large-scale biopharmaceutical production quality control needs. The kit’s flexible reaction capacity and simple operation process make it suitable for small-batch sample testing in research labs, while its high precision, reproducibility, and GMP compliance also meet the strict requirements of large-scale production line high-throughput testing. It can seamlessly integrate into different workflow scenarios without additional equipment adjustments.
Ready to Streamline Your HEK293 Residual DNA Testing?
Whether you’re optimizing gene therapy viral vector production, ensuring recombinant protein compliance, or scaling up HEK293 bioproduction, our resDNASEQ HEK293 Residual DNA Quantitation Kit delivers the sensitivity, efficiency, and reliability your QC team needs. Contact our dedicated sales and technical support team today to request a custom quote, learn more about bulk pricing options, or get personalized guidance on integrating this assay into your GMP workflow. Let us help you meet regulatory standards, reduce testing timelines, and ensure the safety of your biotherapeutic products—reach out now to get started.
Related products
-
Mycoplasma Detection Kit(qPCR)
$1,799.00 -
Vero Residual HCD Quantitation Kit
$1,899.00







