Vero Residual HCD Quantitation Kit
$1,899.00
Ultra-sensitive 0.3fg/μL Vero residual DNA quantitation kit engineered for viral vaccine production QC— the gold standard for African green monkey Vero cell line biopharm manufacturing. Features 20-minute room-temperature sample prep (3 simple steps), 2-hour total TaqMan qPCR test, 0.3fg/μL-300pg/μL broad linear range (R²≈0.9999), 100.627% amplification efficiency, and 70-130% recovery rate. CV <30% for all concentrations, fully suitable for GMP-compliant viral vaccine and biopharm QC testing. Request your custom quote today!
| Catalog number: | D24011204 |
|---|---|
| Qty: | 100Reactions |
Description
Vero host-cell DNA Quantitation Kit from China
Core Unique Advantage
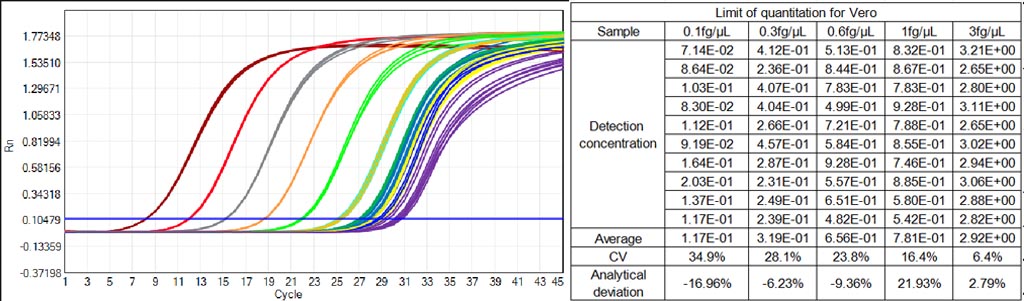
1.Ultra-Sensitive Vero-Specific Quantitation for Vaccines: Achieves an industry-leading Limit of Quantification (LOQ) of 0.3 fg/μL and a broad linear range of 0.3fg/μL to 300pg/μL, with a regression coefficient (R²) nearly 0.9999 and amplification efficiency of 100.627%—ensuring detection of even trace Vero residual DNA in viral vaccine samples, the strictest requirement for vaccine QC. CV values <30% for all concentrations ≥0.3fg/μL and accuracy deviation within 30% guarantee data integrity for regulatory submissions.

2.Two Hours Rapid Workflow with Room-Temp Sample Prep: Reimagines Vero Residual DNA testing for vaccine manufacturing efficiency—only 3 simple steps for sample preparation, completed in just 20 minutes, with all residual DNA sample prep components stable at room temperature. No costly cold chain storage or complex protocols required, and a single ready-to-use qPCR MIX eliminates hands-on error and reduces lab time. The entire assay (sample prep + qPCR amplification + quantitation) is finished in 2 hours—far faster than generic residual DNA kits, accelerating vaccine production QC timelines.


3.Reliable Performance for Real-World Vaccine Samples: Delivers a 70%-130% recovery rate for both simulated and actual Vero cell DNA samples, including low-concentration trace contaminants and high-concentration in-process samples—validating consistent performance in the complex matrices typical of viral vaccine production (e.g., cell culture supernatants, vaccine bulk). Within-run and total precision both show CV <30%, meeting the strict GMP requirements for vaccine quality control and batch-to-batch consistency.






4.User-Friendly Design for GMP Facilities: No specialized equipment or advanced lab training required—compatible with all commercial real-time qPCR instruments, and the single-component qPCR MIX reflects DuckyBio’s advanced formulation technology, minimizing human error and streamlining routine QC testing. Every kit undergoes rigorous batch-to-batch validation, with detailed performance data included to support your viral vaccine regulatory compliance efforts.
Kit Performance & Validated Results
Rigorous experimental validation confirms the kit’s unrivaled performance for Vero residual DNA quantitation in viral vaccine QC scenarios:
- Amplification & Linearity: Seven concentration Vero DNA samples (0.3fg/μL-300pg/μL) show a regression coefficient close to 0.9999 and amplification efficiency of 100.627%, with perfect, reproducible amplification curves for all concentrations.
- Precision & Accuracy: CV values as low as 6.4% for high-concentration samples (3fg/μL) and <30% for all concentrations ≥0.3fg/μL; accuracy deviation within 30% for all tested Vero DNA levels, ensuring reliable quantitation for trace contaminants.
- Recovery Rate: 70%-130% recovery for both simulated Vero DNA samples (0.3fg/μL to 300pg/μL) and actual vaccine production spiked samples, validating performance in real-world bioprocessing matrices.
- Sample Prep Efficiency: 3 simple steps for sample preparation, completed in 20 minutes—no centrifugation or specialized reagents required, with all prep components stable at room temperature for simplified inventory and storage.
Why Choose Our Vero resDNA Quant Kit for Vaccine Production?
Key Applications
- Viral vaccine manufacturing QC (influenza, COVID, polio, and other Vero cell-derived vaccines)
- Vero cell line bioprocess development and scale-up testing
- In-process Vero residual DNA detection (cell culture, purification, formulation)
- Final viral vaccine product residual DNA quantitation
- Bulk vaccine substance quality control for regulatory compliance
- GMP-compliant biopharmaceutical testing for Vero host-cell DNA
- African green monkey Vero cell line characterization and QC
Technical Specifications
| Parameter | Specification |
|---|---|
| Catalog Number | D24011204 |
| Reaction Capacity | 100 Reactions |
| Detection Technology | TaqMan™ Real-Time Quantitative PCR (qPCR) |
| Limit of Quantification (LOQ) | 0.3 fg/μL |
| Linear Dynamic Range | 0.3 fg/μL – 300 pg/μL |
| Regression Coefficient (R²) | ≈0.9999 |
| Amplification Efficiency | 100.627% |
| Recovery Rate | 70% – 130% (simulated & actual samples) |
| Coefficient of Variation (CV) | <30% (all concentrations ≥0.3fg/μL) |
| Sample Preparation Time | 20 Minutes (3 simple steps) |
| Total Assay Time | 2 Hours (sample prep + qPCR + quantitation) |
| Sample Prep Storage | All components stable at room temperature (no freezing/cold storage) |
| qPCR Reagent Feature | Single ready-to-use qPCR MIX |
| Precision | Within-run & total precision CV <30% |
| Sample Compatibility | Vero cell culture supernatants, vaccine bulk, final viral vaccine formulations |
| Compliance | GMP-compliant for biopharm/vaccine manufacturing QC |
You May Also Interested
FAQ
1.What is the LOQ of this Vero residual DNA quantitation kit?
2.How long does a full Vero residual DNA test take with this kit?
3.Is this kit designed specifically for Vero cell line detection?
4.Do the sample preparation components require cold storage?
5.Is this kit suitable for viral vaccine production QC?
6.What is the amplification efficiency of this Vero DNA kit?
Related products
-
Human Residual DNA Fragment Analysis Kit
$3,699.00 -
NS0&SP2/0 HCD resDNA Detection Kit
$1,899.00 -
E.coli Residual DNA Quantitation kit
$1,899.00 -
Pichia Pastoris resDNA Assay Kit
$1,899.00






